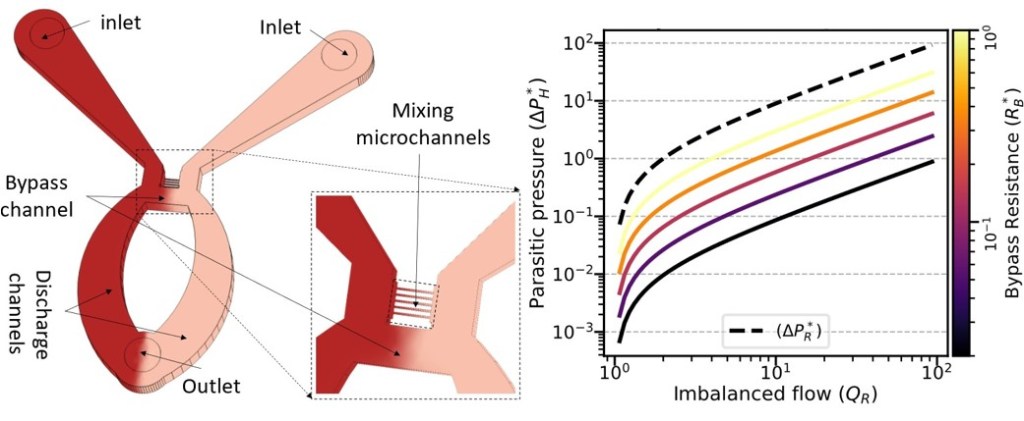
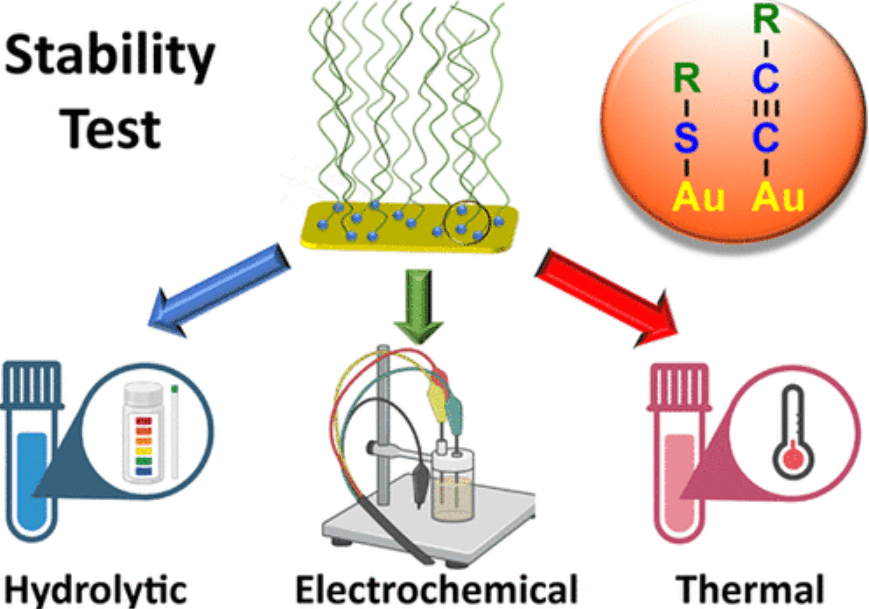
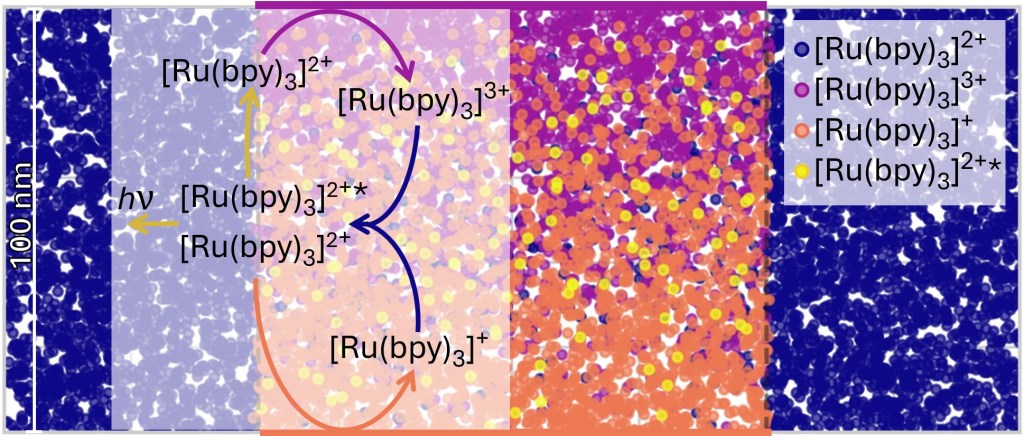
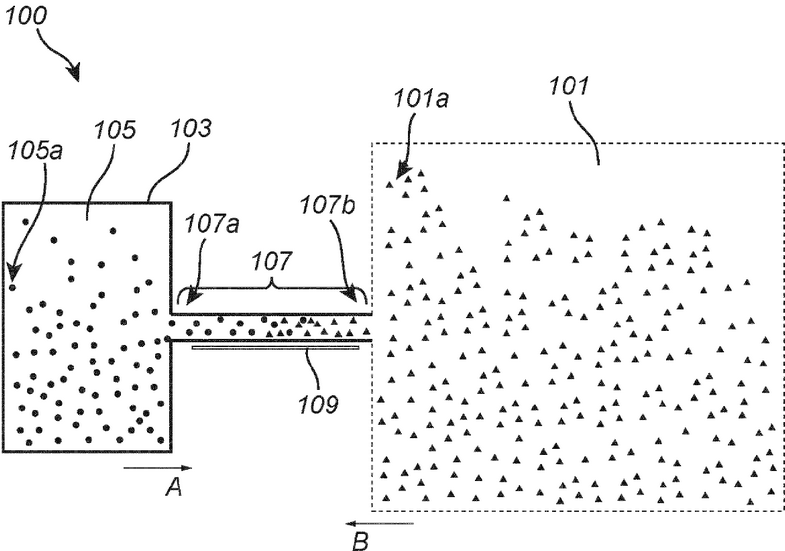
M. Zevenbergen, Y. Abbas, J. Oudenhoven, K. Mathwig, European Patent Application EP4647751A1. [link, pdf]
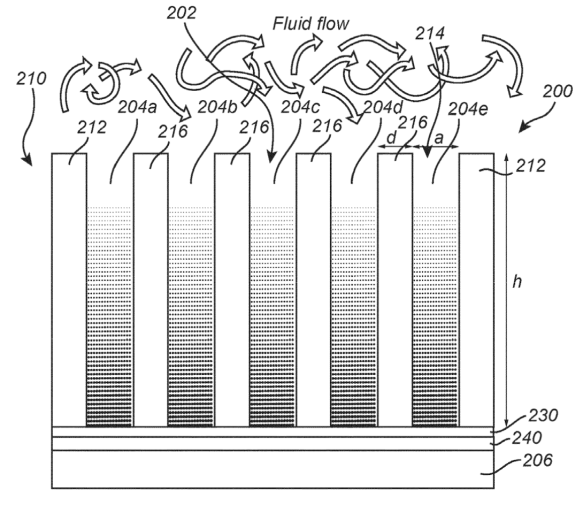
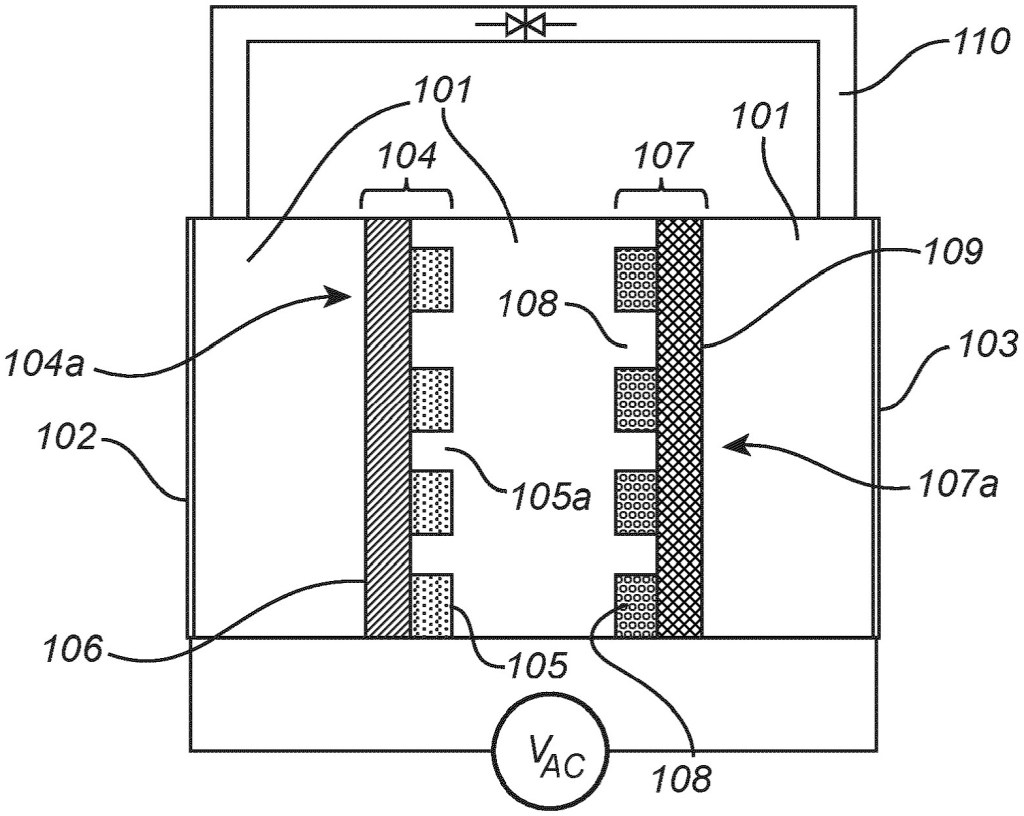
A device for amperometric sensing comprises: an enclosing structure defining a sensing volume and comprising at least one circumferential wall, wherein the enclosing structure defines an inlet in or at an end of the circumferential wall for allowing a target analyte to enter the sensing volume, an electrode arranged in the sensing volume and displaced from the inlet; and a read-out circuitry connected to the electrode and configured for read-out of an electrical signal from the electrode within a time frame during which a target analyte depletion layer around the electrode expands along the circumferential wall and is substantially contained within the sensing volume, wherein the electrical signal is
representative of a concentration of the target analyte.