C. Sharma, S. E. C. Dale, K. Mathwig, M. A. G. Zevenbergen, Z. Li., Bhuvanesh E., K. Parida, Y. S. Negi, F. Marken, ChemElectroChem (2024), e202400411. [link, pdf]
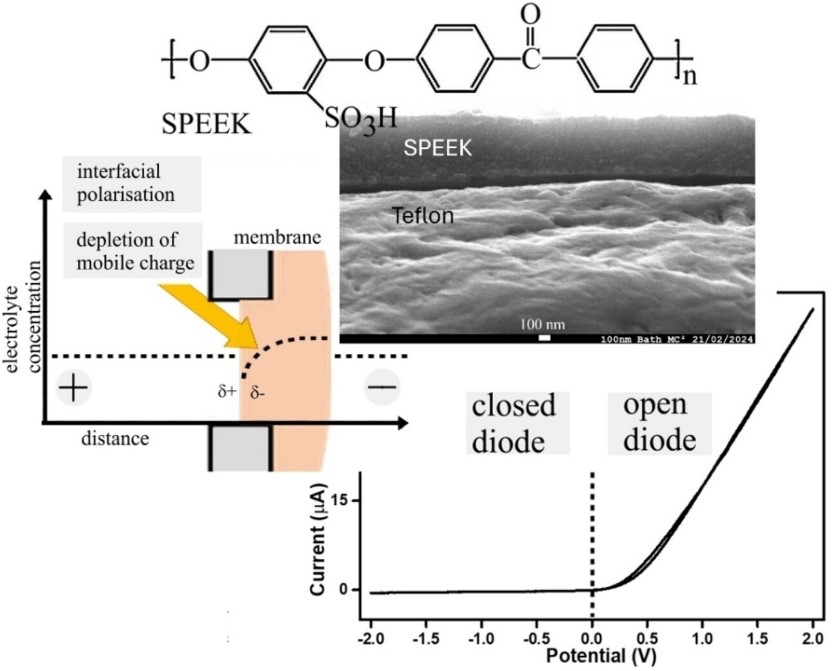
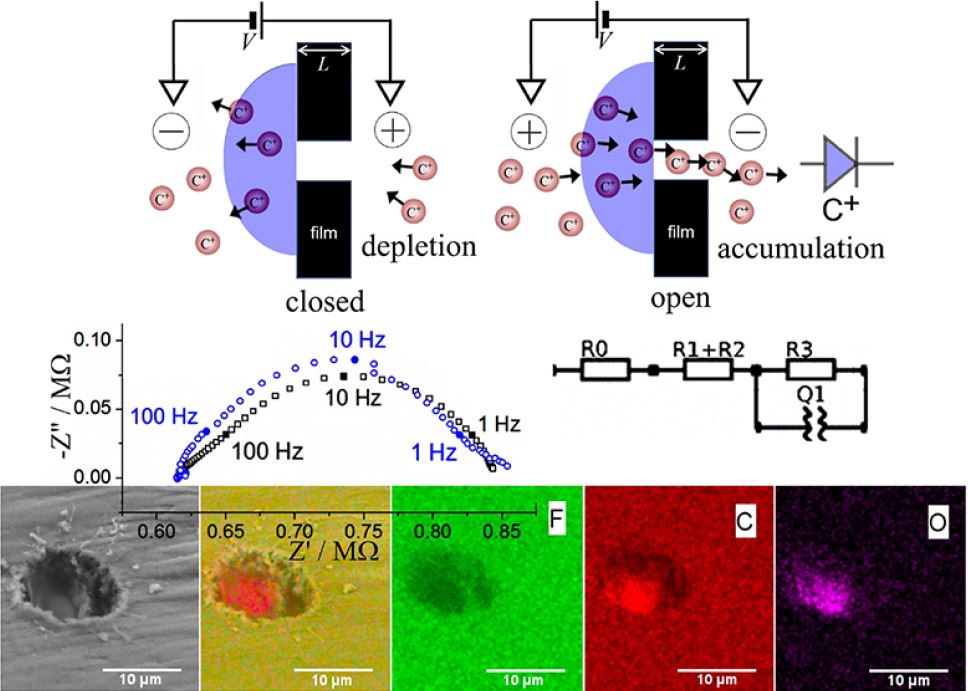
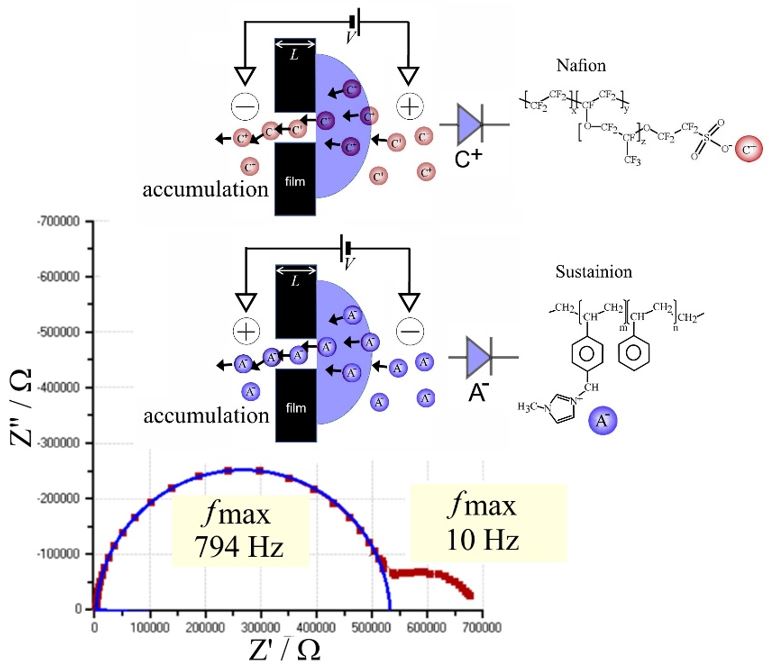
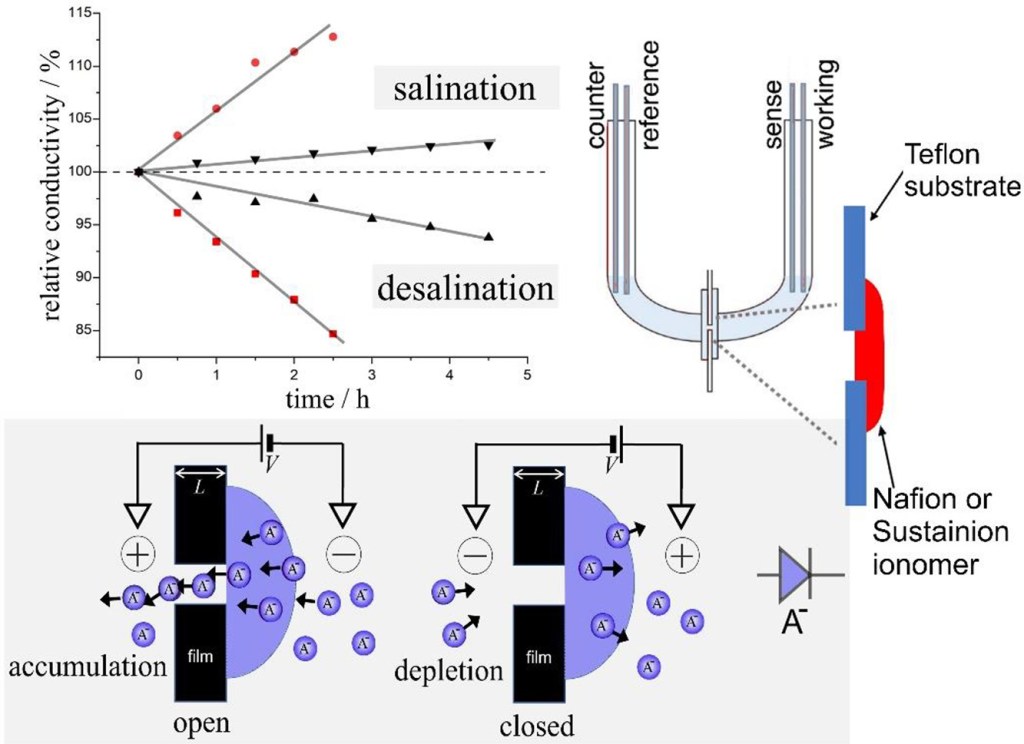
Sulfonated poly(oxy-1,4-phenylene-oxy-1,4-phenylenecarbonyl-1,4-phenylene) also known as SPEEK is a chemically robust cation conductor with good solution processability. A thin film (approx. 0.7 μm) coated asymmetrically over a 10 μm diameter microhole in a Teflon substrate film (5 μm thickness) produces ionic diode effects in aqueous electrolyte media even at high ionic strengths up to 2 M NaCl. The enhancement in the ionic diode performance under high salt conditions is tentatively attributed to a (partial) switch from a concentration polarisation effect (dominant for high diode currents) to interfacial polarisation (dominant at low current; proposed for molecularly rigid ionomers). Ionic strength effects on the diode performance seem relatively low further indicative of a mechanism for the diode effect caused by interfacial polarisation without significant concentration polarisation. Preliminary comparison of diode phenomena in aqueous HCl, LiCl, NaCl, and MgCl2 reveals cation specific effects due to interaction with the polymer.