J. Mestres, F. Leonardi, K. Mathwig, Micromachines 13 (2022) 362. [link, pdf]
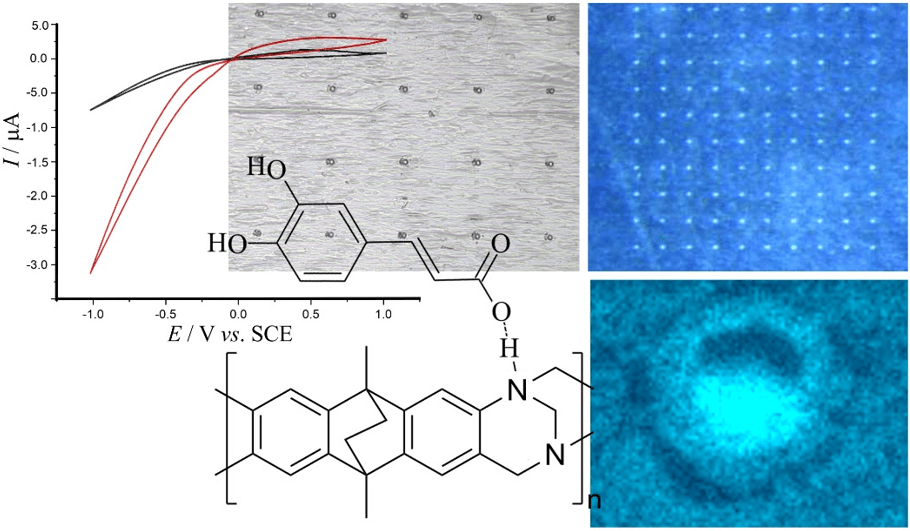
Electrochemical sensors are powerful tools for the detection and real-time monitoring of a wide variety of analytes. However, the long-term operation of Faradaic sensors in complex media is challenging due to fouling. The protection of the electrode surface during in vivo operation is a key element for improving the monitoring of analytes. Here, we study different EUDRAGIT® controlled release acrylate copolymers for protecting electrode surfaces. The dissolution of these polymers—namely EUDRAGIT® L 30 D-55 and EUDRAGIT® FS 30 D—is triggered by a change in pH of the environment, and it is electrochemically monitored by detecting electrode access by means of a redox probe. The full dissolution of the polymer is achieved within 30 min and the electrode response indicates a complete recovery of the original electrochemical performance. We demonstrate that amperometric sensing is a practical and straightforward technique for real-time and in situ sensing of EUDRAGIT® dissolution profiles. It will find future applications in determining the protection of polymer electrode coating in real matrices and in vivo applications.


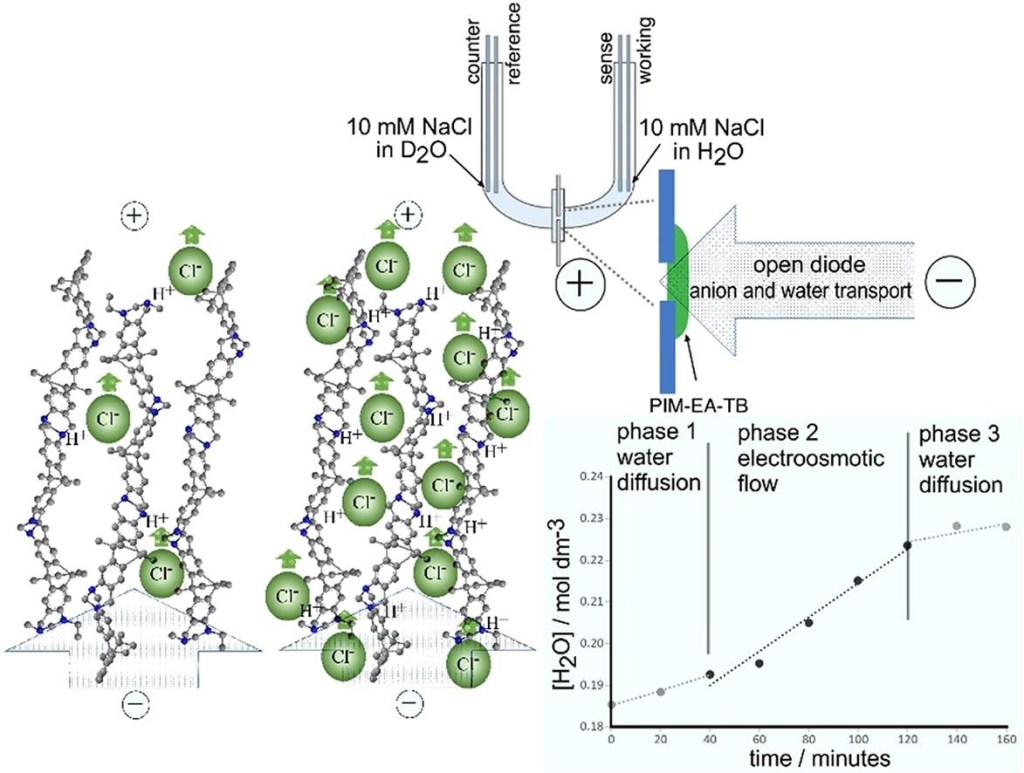
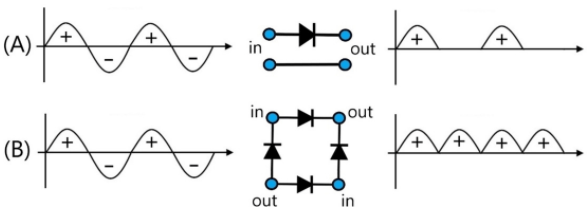
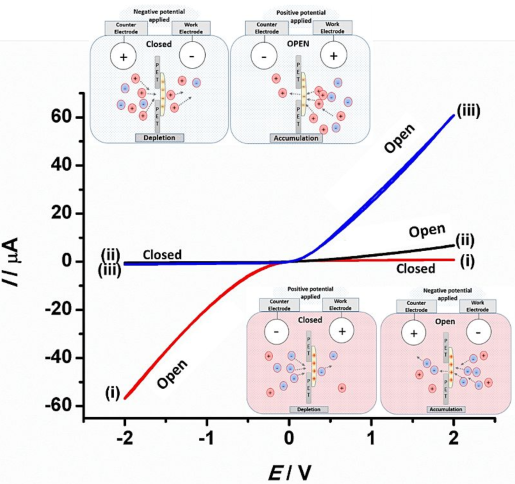 Membrane materials with semi-permeability for anions or for cations are of interest in electrochemical and nanofluidic separation and purification technologies. In this study, partially hydrolyzed poly-acrylonitrile (phPAN) is investigated as a pH-switchable anion/cation conductor. When switching from anionic to cationic semi-permeability, also the ionic current rectification effect switches for phPAN materials deposited asymmetrically onto a 5, 10, 20, or 40 µm diameter microhole in a 6 µm thick polyethylene-terephthalate (PET) film substrate. Therefore, ionic rectifier behavior can be tuned and used to monitor and characterize semi-permeability. Effects of electrolyte type and concentration, and pH (relative to the zeta potential at approximately 3.1) are investigated by voltammetry, chronoamperometry, and impedance spectroscopy. A computational model provides good qualitative agreement with observed electrolyte concentration data. High rectification effects are observed for both cations (pH > 3.1) and anions (pH < 3.1), but only at relatively low ionic strengths.
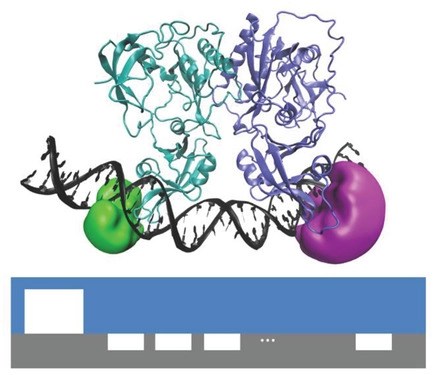
Membrane materials with semi-permeability for anions or for cations are of interest in electrochemical and nanofluidic separation and purification technologies. In this study, partially hydrolyzed poly-acrylonitrile (phPAN) is investigated as a pH-switchable anion/cation conductor. When switching from anionic to cationic semi-permeability, also the ionic current rectification effect switches for phPAN materials deposited asymmetrically onto a 5, 10, 20, or 40 µm diameter microhole in a 6 µm thick polyethylene-terephthalate (PET) film substrate. Therefore, ionic rectifier behavior can be tuned and used to monitor and characterize semi-permeability. Effects of electrolyte type and concentration, and pH (relative to the zeta potential at approximately 3.1) are investigated by voltammetry, chronoamperometry, and impedance spectroscopy. A computational model provides good qualitative agreement with observed electrolyte concentration data. High rectification effects are observed for both cations (pH > 3.1) and anions (pH < 3.1), but only at relatively low ionic strengths. In ion-annihilation electrochemiluminescence (ECL), luminophore ions are generated by oxidation as well as reduction at electrodes surfaces, and subsequently recombine into an electronically excited state, which emits light. The intensity of the emitted light is often limited by the kinetic rate of recombination of the luminophore ion species. Recombination or annihilation rates are high ranging up to approximately 1010 M−1 s−1 and can be difficult to determine using scanning electrochemical microscopy or high-frequency oscillations of an electrode potential. Here, we propose determining annihilation kinetics by measuring the relative change of the emitted light intensity as a function of luminophore concentration. Using finite element simulations of annihilation ECL in a geometry of two closely spaced electrodes biased at constant potentials, we show that, with increasing concentrations, luminescence intensity crosses over from a quadratic dependence on concentration to a linear regime—depending on the rate of annihilation. Our numerical results are applicable to scanning electrochemical microscopy as well as nanofluidic electrochemical devices to determine fast ion-annihilation kinetics.
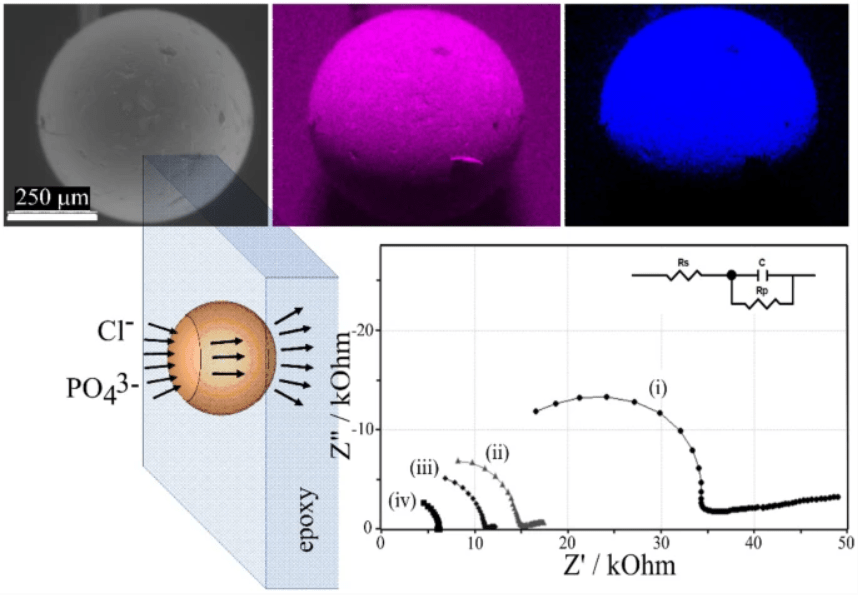
In ion-annihilation electrochemiluminescence (ECL), luminophore ions are generated by oxidation as well as reduction at electrodes surfaces, and subsequently recombine into an electronically excited state, which emits light. The intensity of the emitted light is often limited by the kinetic rate of recombination of the luminophore ion species. Recombination or annihilation rates are high ranging up to approximately 1010 M−1 s−1 and can be difficult to determine using scanning electrochemical microscopy or high-frequency oscillations of an electrode potential. Here, we propose determining annihilation kinetics by measuring the relative change of the emitted light intensity as a function of luminophore concentration. Using finite element simulations of annihilation ECL in a geometry of two closely spaced electrodes biased at constant potentials, we show that, with increasing concentrations, luminescence intensity crosses over from a quadratic dependence on concentration to a linear regime—depending on the rate of annihilation. Our numerical results are applicable to scanning electrochemical microscopy as well as nanofluidic electrochemical devices to determine fast ion-annihilation kinetics.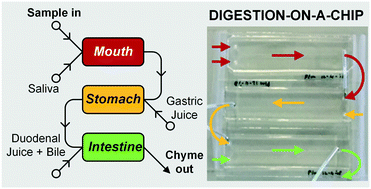 In vitro digestions are essential for determining the bioavailability of compounds, such as nutrients. We have developed a cell-free, miniaturized enzymatic digestive system, employing three micromixers connected in series to mimic the digestive functions of the mouth, stomach and small intestine. This system continuously processes samples, e.g. containing nutrients, to provide a constant flow of digested materials which may be presented to a subsequent gut-on-a-chip absorption module, containing living human intestinal cells. Our system incorporates three-compartment enzymatic digestion, one of the key functions of the gastrointestinal tract. In each of these compartments, we modify the chemical environment, including pH, buffer, and mineral composition, to closely mimic the local physiological environment and create optimal conditions for digestive processes to take place. It will therefore provide an excellent addition to existing gut-on-a-chip systems, providing the next step in determining the bio-availability of orally administered compounds in a fast and continuous-flow ex vivo system. In this paper, we demonstrate enzymatic digestion in each separate compartment using compounds, starch and casein, as model nutrients. The use of transparent, microfluidic micromixers based on chaotic advection, which can be probed directly with a microscope, enabled enzyme kinetics to be monitored from the very start of a reaction. Furthermore, we have digested lactoferrin in our system, demonstrating complete digestion of this milk protein in much shorter times than achievable with standard in vitro digestions using batch reactors.
In vitro digestions are essential for determining the bioavailability of compounds, such as nutrients. We have developed a cell-free, miniaturized enzymatic digestive system, employing three micromixers connected in series to mimic the digestive functions of the mouth, stomach and small intestine. This system continuously processes samples, e.g. containing nutrients, to provide a constant flow of digested materials which may be presented to a subsequent gut-on-a-chip absorption module, containing living human intestinal cells. Our system incorporates three-compartment enzymatic digestion, one of the key functions of the gastrointestinal tract. In each of these compartments, we modify the chemical environment, including pH, buffer, and mineral composition, to closely mimic the local physiological environment and create optimal conditions for digestive processes to take place. It will therefore provide an excellent addition to existing gut-on-a-chip systems, providing the next step in determining the bio-availability of orally administered compounds in a fast and continuous-flow ex vivo system. In this paper, we demonstrate enzymatic digestion in each separate compartment using compounds, starch and casein, as model nutrients. The use of transparent, microfluidic micromixers based on chaotic advection, which can be probed directly with a microscope, enabled enzyme kinetics to be monitored from the very start of a reaction. Furthermore, we have digested lactoferrin in our system, demonstrating complete digestion of this milk protein in much shorter times than achievable with standard in vitro digestions using batch reactors.